APO-Easy® Genotyping Kit
INSTRUCTION FOR USE
![]() FLS-OE-02
FLS-OE-02
For in vitro Diagnostic Use only
Not for therapeutic use
Version: 3.1
Date of Issue:21/05/24
Download here the PDF of the IFU:
REC_RGA_109_40116_IFU-APO-Easy-genotyping-kit-FLS-OE-02_final.pdf (amoneta-diagnostics.com)
![]()
Firalis SA Phone: +00 33 389 911 320 35 rue du Fort Email: contact@firalis.com 68330 – Huningue FRANCE Web: www.firalis.com
![]()
32
|
Contents: |
|
1. Intended Use..................................................................................................................................................................................................5 |
|
3. Principle of the assay ..................................................................................................................................................................................6 |
|
4. Instruments and materials.........................................................................................................................................................................6 |
|
4.1 Reagents and materials provided with the kit ...................................................................................................................................8 |
|
4.3 Kit storage requirements and shelf-life of the kit ............................................................................................................................8 |
|
5. Genomic DNA (gDNA) extraction & quantification ...............................................................................................................................8 |
|
6. APO-Easy® Genotyping assay procedure ...............................................................................................................................................8 |
|
6.1 Step 1. Real-time genotyping PCR reaction preparation .................................................................................................................9 |
|
6.2 Step 2. qPCR run .....................................................................................................................................................................................10 |
|
6.3 Step 3 Result analysis.............................................................................................................................................................................12 |
|
7. Troubleshooting ..........................................................................................................................................................................................15 |
|
9. Clinical Performance..................................................................................................................................................................................17 |
|
10. Residual Risks and warnings...................................................................................................................................................................17 |
|
11. Limitations of the Procedure...................................................................................................................................................................19 |
|
12. Chemical safety guidelines.....................................................................................................................................................................20 |
|
13. Technical hints...........................................................................................................................................................................................20 |
|
14. Liability.......................................................................................................................................................................................................20 |
|
Technical assistance....................................................................................................................................................................................... 21 |
|
Serious incident report notice .................................................................................................................................................................... 21 |
|
Summary of Safety and Performance......................................................................................................................................................... 21 |
|
References........................................................................................................................................................................................................22 |
|
Symbol legend used in the IFU and labels of the kit..............................................................................................................................23 |
Tables:
Table 5. Model layout of a 96 well plate for APOE genotyping of 24 samples, 3 controls/SNP in duplicate and 2 NTCs/SNP for APO-Easy® Genotyping kit...................................................................................................................................................................... 10
Table 10. APO-Easy genotyping test has 100% accuracy ......................................................................................................................... 17
Table 11 Genotype based comparisons between AD and NAD + HC .......................................................................................................17
Figures:
List of abbreviations:
SNP: single nucleotide polymorphisms
gDNA: genomic deoxyribonucleic acid
APOE: Apolipoprotein E
AD: Alzheimer’s Disease
qPCR: qualitative real-time Polymerase Chain Reaction
IVD: In Vitro Diagnostic
QS5-Dx: QuantStudio 5 Dx Real-Time PCR System
NTC: No Template Control
MUT: Mutant
WT: Wild Type
Het: Heterozygous
RCF: Relative Centrifugal Force
°C: Celsius degree
ng: nanogram
µL: microliter
STD: standard
IFU: Instruction For Use
SDS: Safety Data Sheets
1. Intended Use
APO-Easy® genotyping kit is a qualitative in vitro diagnostic test intended for the detection of two single nucleotide polymorphisms (SNPs) rs429358 and rs7412 in human genomic DNA. It enables the determination of six Apolipoprotein E (APOE) genotypes: e2/e2, e2/e3, e3/e3, e2/e4, e3/e4, and e4/e4 based on RT-PCR. The APO-Easy® genotyping test result is intended to be used by physicians, in conjunction with other clinical examinations, to aid in evaluating an individual’s level of risk of developing Alzheimer’s Disease (AD). An individual with ε2 alleles exhibits the lowest risk for developing AD, indicative of its protective effect. The presence of ε3 alleles is observed mainly in normal individuals and does not alter the risk of developing AD. An individual with a single ε4 allele (heterozygous e2/e4, or e3/e4) is associated with a high risk of developing AD, compared to the other APOE genotypes without e4 (e2/e2, e2/e3, and e3/e3). The presence of double ε4 alleles (homozygous e4/e4) is associated with the highest risk of developing AD, compared to all other APOE genotypes.
Conditions for Use:
• For prescription use
• For use in adult humans (> 18 years old)
• For use in molecular diagnostics laboratories by qualified operators
• This test is not an automated method
• The test cannot be carried out by the patient at home. It is neither a point-of-care device nor a self-administered test device
• This test is not intended to replace any clinical and diagnostic examinations.
2. Introduction
Apolipoprotein E (APOE) is a chylomicron apolipoprotein expressed in various organs such as the liver, brain, spleen, kidneys, sex glands, adrenal glands, and macrophages (Marias, 2019). APOE has been shown to be important for various processes such as the metabolism of lipoproteins, fat-soluble vitamins, and glucose/energy as well as signal transduction, metastasis, and angiogenesis. Being important in lipid metabolism, APOE is well-linked to cardiovascular disease as it is required for the normal catabolism of the triglyceride-rich lipoprotein components (Semaev et al., 2022). The importance of APOE in the pathogenesis of neurodegenerative disorders such as frontotemporal dementia, Parkinson’s disease, Lewy body dementia, and Alzheimer’s disease (AD) has been well documented (Davis et al., 2020; Mishra et al., 2017).
APOE gene has three common alleles (ε2, ε3 and ε4) and six related genotypes (ε3ε3, ε3ε2, ε2ε2, ε3ε4, ε4ε4, and ε2ε4) [Su et al., 2017]. The corresponding isoforms are characterized by amino acids at positions 112 and 158 and determine the affinity for lipoprotein receptors. APOE ε3 is the most common allele believed neither to increase nor decrease the risk of developing AD. APOE ε2 has been shown to have a protective effect against developing AD (Ohm et al., 1999) whereas APOE ε4 reduced the clearance of beta-amyloid (Aβ) that resulted in enhanced Aβ deposition within the neurons in the AD mouse model (Yamazaki et al., 2019). About 25 percent of people carry one copy of APOE ɛ4, and 2 to 3 percent carry two copies (https://www.nia.nih.gov/health/alzheimers-disease-genetics-fact-sheet). Mayeux et al., 1998 studied 2188 AD patients and showed that when combined with clinical criteria, the presence of APOE ε4 genotype improves the specificity of AD diagnosis. Several studies have evidenced that the APOE ε4 genotype is associated with late onset of familiar and sporadic forms of AD (Corder et al., 1993, Schmechel et al., 1993, Strittmatter et al., 1993, Farrer et al., 1997, Bu G., 2009).
The APO-Easy® Genotyping kit uses qualitative real-time polymerase chain reaction (qPCR) technique for the in vitro determination of two APOE single-nucleotide polymorphisms (SNPs) mutation: rs429358 and rs7412. This IVD test uses fluorescently labelled probes (FAM and VIC) allowing allelic discrimination and determination of APOE genotypes.
3. Principle of Assay
The APO-Easy® Genotyping kit includes reagents needed to perform qPCR analysis for testing genomic DNA (gDNA) extracted from PAXgene DNA samples to determine APOE genotypes. The assay assesses 2 SNPs (rs429358 and rs7412) in the APOE gene and contains positive control for each SNPs (wild-type, mutant and heterozygous). The genotype is determined based on the mutation status for both mutations analyzed resulting in one of the six genotypes possible (ε3ε3, ε3ε2, ε2ε2, ε3ε4, ε4ε4, and ε2ε4).
For the wildtype allele (ApoE3) the amino acids cysteine at position 112 and arginine at position 158 are detected. The mutation ApoE4 (SNP rs429358) impacts the amino acid at position 112 resulting in changing thymine to cytosine inducing the translation of arginine instead of cysteine. Whereas the mutation ApoE2 (SNP rs7412) impacts the amino acid at position 158 resulting in changing cytosine to thymine inducing the translation of cysteine instead of arginine (Figure 1).

Figure 1. Schematic diagram of human APOE gene and the polymorphisms at 2 single nucleotides resulting in 3 alleles ε2, ε3 and ε4.
4. Instruments and Material
The APO-Easy® Genotyping kit includes consumables to perform the qPCR amplification and fluorogenic detection of APOE mutations using ThermoFisher QS5-Dx (equipment not provided with the kit). The equipment needed for the assay but not provided with the kit and their references are given in Table 1.
TabTTable 1. Reference of the equipment needed to perform APO-Easy® Genotyping kit
|
Instrument |
Purpose |
Manufacturer |
Reference no. |
Certifications |
|
Nanodrop 2000/2000c |
Quantification of gDNA |
ThermoFischer |
ND-2000 |
UL/CSA and CE |
|
QS5-Dx |
qPCR |
ThermoFischer |
12014330 |
FDA 510K (K190302) |
For gDNA extraction from whole blood PAXgene DNA tubes, Firalis recommends using the reference given in table 2 following the manufacturer instruction provided along with the kit.
If another reference is used, Firalis cannot guarantee the results nor performance of the assay. Samples must be collected in PAXgene® Blood DNA Tube (BD Biosciences, Reference no: 761165).
Table 2. Reference of the recommended gDNA extraction kit (not provided along with the APO-Easy® Genotyping kit).
|
Description |
Manufacturer |
Purpose |
Storage |
Reference |
Quantity |
|
QIAamp DSP DNA Blood Mini kit |
Qiagen |
gDNA extraction |
According to the supplier’s recommendation |
61104 |
1kit/50samples |
4.1 Reagents and materials provided with the kit
The APO-Easy® Genotyping kit contains ready-to-use master mixes for 32 reactions per SNP, positive controls: Standard A, Standard B, Standard C, Standard D, Standard E, and one vial of no template control (NTC). A total of 32 reactions is possible using the reagents from one kit including 6 reactions for the standards and 2 NTCs for each SNP, and for each analysis (Table 3).
Table 3. Contents of the APO-Easy® Genotyping kit.
|
Description |
Storage |
Reference |
Quantity |
|
TaqMan™ Genotyping Master Mix 1 rs429358 |
-15°C to -25°C |
FLS-MM1OE-02 |
1 vial |
|
TaqMan™ Genotyping Master Mix 2 rs7412 |
-15°C to -25°C |
FLS-MM2OE-02 |
1 vial |
|
No Template Control (NTC) |
-15°C to -25°C |
FLS-NTC-01 |
1 vial |
|
Standard A: Mutant (MUT) rs7412 |
-15°C to -25°C |
FLS-OE2S-01 |
1 vial |
|
Standard B: Wildtype (WT) rs429358 and rs7412 |
-15°C to -25°C |
FLS-OE3S-01 |
1 vial |
|
Standard C: Mutant (MUT) rs429358 |
-15°C to -25°C |
FLS-OE4S-01 |
1 vial |
|
Standard D: Heterozygous (Het) rs7412 |
-15°C to -25°C |
FLS-OE23S-01 |
1 vial |
|
Standard E : Heterozygous (Het) rs429358 |
-15°C to -25°C |
FLS-OE34S-01 |
1 vial |
*Positive controls: Standard A, Standard B, Standard C, Standard D, Standard E. Negative control: NTC
4.2. Miscellaneous materials and equipment needed
- Nuclease-free water
- Use only certified nuclease-free tips and microcentrifuge tubes
- Precision pipettes - Multichannel pipette
- BSL2 cabinet
- Micro centrifuge (17000 Relative Centrifugal Force (RCF))
- Vortex and microcentrifuge
- Real-time PCR plates
- Compact PCR plates spinner
4.3 Kit storage requirements & shelf-life of the kit
- The kit must be stored at -20°C.
- Kit components may be repeatedly thawed and frozen twice and remain stable. Exciding the freeze and thaw cycle recommended might diminuish the kit functionality.
- The kit is stable until its expiration date stated on the box label.
5. Genomic DNA (gDNA) extraction and quantification
5.1 Extraction
Extraction of gDNA from whole human blood collected into PAXgene Blood DNA tubes using QIAamp DSP DNA Blood Mini kit as given in table 2 following the user instruction data sheet provided along with the kit.
5.2 Quantification
Quantification of the extracted gDNA is done using Nanodrop. The samples are to be adjusted to have 6.25 ng/µL from which 2 µL (12.5 ng) will be used for the assay. If the samples have concentration higher than the recommended concentration, they must be diluted in Nuclease Free water (Molecular grade). If the samples have lower concentration than the required amount, it is recommended to use 2 µL in total. The amount of gDNA input used for the APO-Easy® Genotyping assay is 12.5 ng.
6. APO-Easy® Genotyping assay procedure
The overview of the APO-Easy genotyping assay workflow is presented in Table 4. The required time for the assay is : 2h30.
Table 4. Overview of the APO-Easy genotyping assay workflow.
Workflow |
|
|
Step 1 |
Real-time genotyping PCR reaction preparation |
|
Step 2 |
qPCR run |
|
Step 3 |
Result analysis |
|
Step 4 |
Interpretation |
6.1 Step 1. Real-time genotyping PCR reaction preparation
The kit comprises of two ready-to-use TaqMan™ Genotyping Master Mix to assess APOE SNPs (rs429358 and rs7412), nuclease-free water for the NTC, and standards to mimic wildtype, mutant and heterozygous conditions for each single nucleotide polymorphisms (SNP).
Each master mix is a dualplex assay assessing the SNP of interest:
Master mix 1: composed of 2 probes to assess the SNP rs429358 and allow allelic discrimination for the wildtype nucleotide (T), the mutant nucleotide (C) as well as the heterozygote condition.
Master mix 2: composed of 2 probes to assess the SNP rs7412 and allow allelic discrimination for the wildtype nucleotide (C), the mutant nucleotide (T) as well as the heterozygote condition.
Standard A, B, C, D, and E:
Each standard (STD) is a DNA template designed to mimic the three alleles and the combination of different DNA templates mimicking the heterozygous condition.
Standard A: DNA template of allele ApoE2
Standard B: DNA template of allele ApoE3
Standard C: DNA template of allele ApoE4
Standard D: DNA template with allele ApoE2 and ApoE3
Standard E: DNA template with allele ApoE3 and ApoE4
Note: Thaw the master mixes and the standards on ice. Mix the reagents thoroughly and spin them down briefly and place them on ice.
- Dispense 23 µL/well of the detection assay master mix in a 96-well plate (for each sample master corresponding to both SNPs must be added).
2.Add 2 µL of gDNA or the Standards in the corresponding wells and briefly spin down. The total amount of gDNA in a reaction should be 12.5 ng.
- Each individual SNP requires analysis of WT, MUT, and HET standards as well as NTC (nuclease free Water molecular grade). A model layout plan for the analysis of up to 24 PAXgene DNA samples measured for 2 SNPs in a 96 wells PCR plate is shown in Table 5.
- Note: APO-Easy genotyping kit allows to perform up to 32 qPCR reactions. Each master mix is for 8 STD and up to 24 samples. If a smaller number of samples are to be assessed the kit can be used twice, for example 8 STD plus 8 patients can be assessed once and the second time again 8 STD plus 8 patients can be assessed.
Table 5. Model layout of a 96 well plate for APOE genotyping of 24 samples, 3 controls/SNP in duplicate and 2 NTCs/SNP for APO-Easy® Genotyping kit.
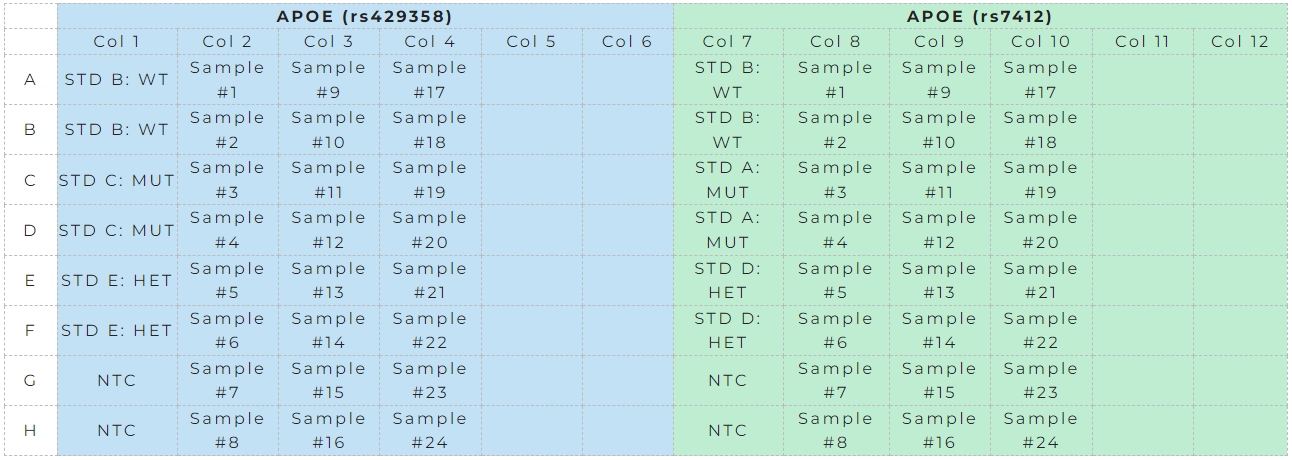
- The following STD must be used for each SNP assay determination as given below. For rs429358 (Master mix 1), standards B, C and E must be used and to assess rs7412 (Master mix 2) standards B, A and D must be used (Table 6).
Table 6. Standard correspondence for SNP determination of the APO-Easy genotyping assay.
|
APOE (rs429358) |
APOE (rs7412) |
|
STD B (WT) |
STD B (WT) |
|
STD C (MUT) |
STD A (MUT) |
|
STD E (HET) |
STD D (HET) |
- Vortex the plate for 5 sec and centrifuge at 500g for 30 seconds.
4.Proceed to start the run immediately.
6.2 Step 2.qPCR run
5. Setting the QS5 Dx for APO-Easy genotyping assay:
A.On “Properties” page, assign experiment name and select the experiment type as genotyping (Figure 2).
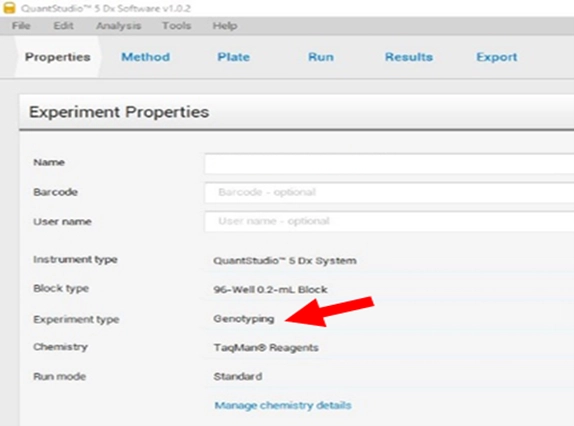
Figure 2. Experiment type selection in the experiment properties on the QS5 software. Make sure to select genotyping (red arrow).
B. On “Method” page, set up the total volume of PCR reaction, 25 µL of reaction mix.
C. On “Plate sheet,” go in advanced Setup, to set up fluorescence, click on “(+) Add button” to obtain two SNP assay (SNP Assay 1 & SNP Assay 2). Click on OK button to proceed. Then for each SNP Assay, click on the Action button and select Edit to fill the name as following:
NOTE:
For SNP Assay 1: rs429358 (Master mix 1). Make sure to have Allele 1 set with the reporter VIC and Allele 2 set with the reporter FAM
For SNP Assay 2: rs7412 (Master mix 2). Make sure to have Allele 1 set with the reporter VIC and Allele 2 set with the reporter FAM
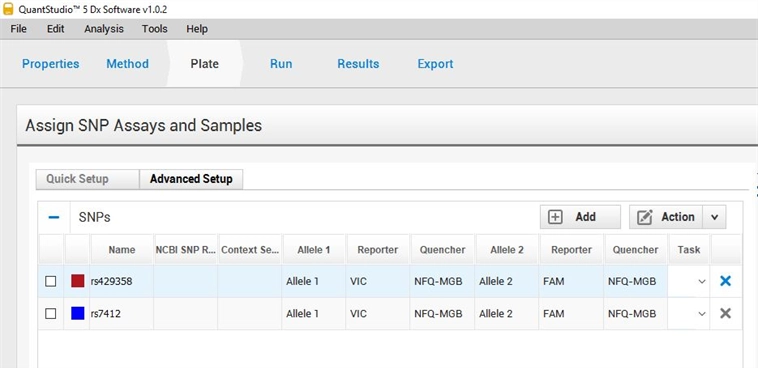
Figure 3. SNP setup for the APO-Easy genotyping kit on the QS5 software.
6. To define Standards for rs429358 (Master mix 1), select column 1 in the plate design and select SNP Assay1 (rs429358). Then, select A1 and B1 (“STD B (WT)”) and assign 2/2 in the task bar. Select C1 and D1 (“STD C (MUT)”) and assign 1/1 in the task bar. Select E1 and F1 (“STD E (HET)”) and assign 1/2 in the task bar. Select G1 and H1 and assign N in the task bar “NTC”.
7. To define Standards for rs7412 (Master mix 2), select column 7 in the plate design and select SNP Assay2 (rs7412). Then, select A7 and B7 (“STD B (WT)”) and assign 1/1 in the task bar. Select C7 and D7 (“STD A (MUT)”) and assign 2/2 in the task bar. Select E7 and F7 (“STD D(HET)”) and assign 1/2 in the task bar. Select G7 and H7 and assign N in the task bar “NTC”.
8. To define Samples for rs429358 (Master mix 1), select columns 2 to 4 in the plate design and select SNP Assay1 (rs429358).
9. To define Samples for rs7412 (Master mix 2), select columns 8 to 10 in the plate design and select SNP Assay2 (rs7412).
10. Once the plate ready and the set up done, go to “Run” sheet and the start the run.
11. The qPCR run program will be automatically select by the QS5-Dx and the program specification should be as indicated in Table 7 :
Table 7. qPCR run program in the QS5 device.
|
Program |
Temperature °C |
Time |
Cycles |
|
Activation |
95 |
10 min |
|
|
Denaturation |
95 |
15 sec |
40 |
|
Extension |
60 |
1 min |
6.3. Step 3. Result analysis
Before the sample result analysis, verify the signal obtained for STD with the respective detection assay in allelic discrimination mode. The qPCR run results can be found on the results page of the QS5 Dx software (Figure 4). In this page select Allelic Discrimination (red arrow). The analysis validity should be evaluated for each mutation separately. The selection of rs429358 or rs7412 can be done by clicking on the icon highlighted by the green arrow.
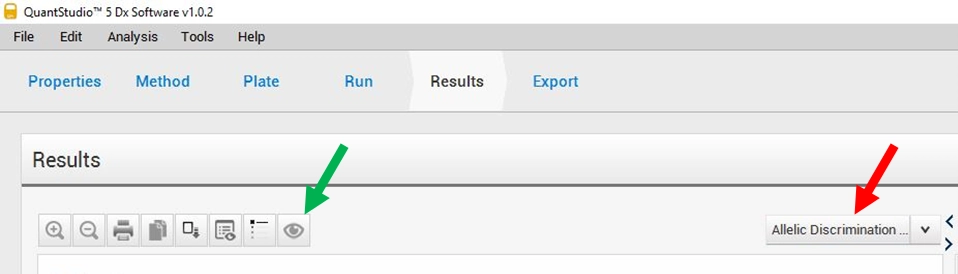
FiFigure 4. qPCR run results page on the QS5 software. The red arrow indicates the type of result to select (Allelic Discrimination) and the green arrow indicates the icon to select the SNPs.
For the STD and NTC, analysis can proceed when the STD signals correspond to the following example. Make sure your standards have a similar pattern as shown in Figure 3. For rs429358 the wild-type (WT) will have the signal on the top left region of the graph in the y-axis (allele 2) corresponding to the homozygous allele2/allele2 (e3). The mutant (MUT) standard will have signal close to the x-axis (allele 1) at bottom right area of the graph corresponding to the allele1/allele1 (e4). The heterozygous standard will be in between MUT and WT corresponding to the heterozygous allele1/allele2 (e3/e4) as shown in Figure 3. NTC will not generate any signal and will be found at the bottom left of the graph.
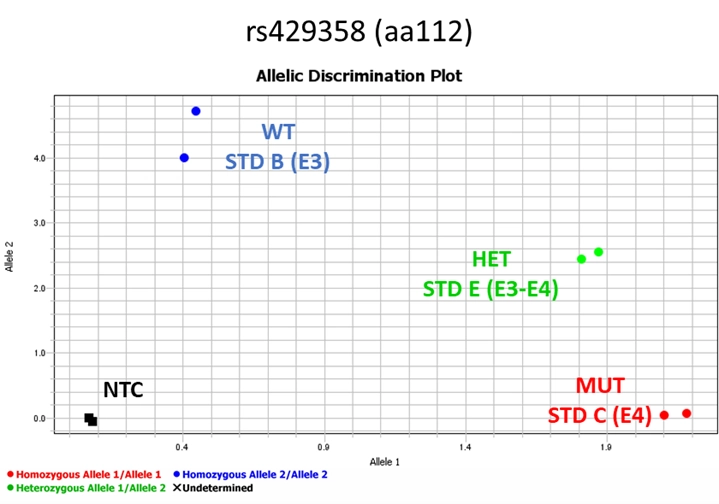
Figure 5. Pattern of the signals of the standards for SNP rs429358 (Master mix 1) after qPCR analysis.
For rs7412 the wild type (WT) will have the signal on the bottom right close to x-axis region of the graph corresponding to the homozygous allele1/allele1 (e3). The mutant (MUT) standard will have a signal to the top left area of the y-axis of the graph corresponding to the allele2/allele2 (e2). The heterozygous standard will be in between MUT and WT corresponding to the heterozygous allele1/allele2 (e2/e3) as shown in Figure 6. NTC will not generate any signal and will be found at the bottom left of the graph.
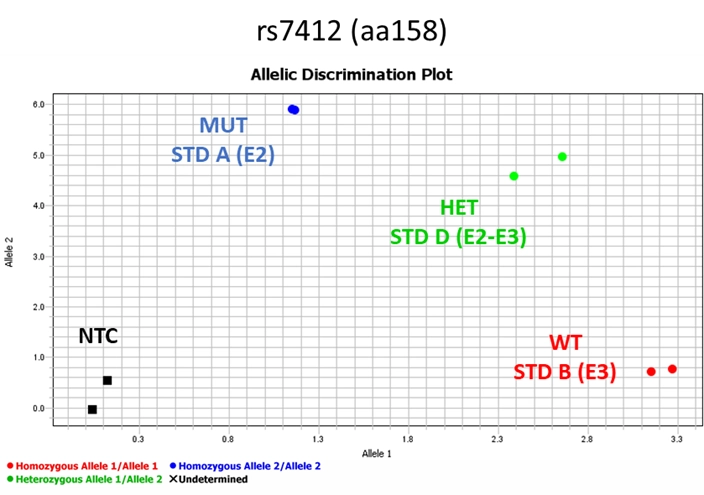
Figure 6. Pattern of the signals of the standards for SNP rs7412 (Master mix 2) after qPCR analysis.
6.4. Step 4. Interpretation
Once the controls are validated, the sample signals are interpreted by clicking on the icon highlighted by the red arrow. For each SNP, patient status is available in the column “Call” (green arrow; Figure 7).
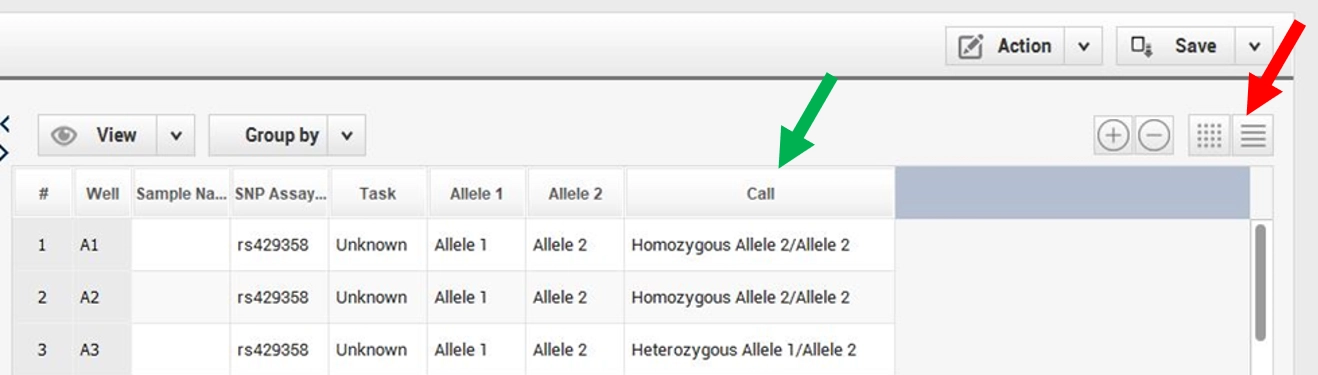
Figure 7. Interpretation of the results on the QS5 software. The red arrow indicates the icon which interpret the signal and the green arrow indicates the patient status for each SNP determined by the software after the results analysis.
For rs429358, Homozygous Allele 2 / Allele 2 corresponds to WT, Homozygous Allele 1 / Allele 1 corresponds to MUT and Heterozygous Allele 1 / Allele 2 corresponds to HET.
For rs7412, Homozygous Allele 1 / Allele 1 corresponds to WT, Homozygous Allele 2 / Allele 2 corresponds to MUT and Heterozygous Allele 1 / Allele 2 corresponds to HET.
Once assigned the status for each SNP, the genotype of the sample can be determined using the Table 8 given below.
Figure 8. Interpretation of genotype from SNPs rs429358 and rs7412 results.
|
rs429358 |
rs7412 |
Genotype |
|
WT |
MUT |
e2/e2 |
|
WT |
HET |
e2/e3 |
|
WT |
WT |
e3/e3 |
|
HET |
HET |
e2/e4 |
|
MUT |
WT |
e4/e4 |
|
HET |
WT |
e3/e4 |
7. Troubleshooting
- Assessment of the test results should be performed after the positive and negative controls have been examined and determined to be valid and acceptable. If the controls are not valid, the Sample results cannot be interpreted.
- If assay failure is encountered:
- Check the expiration dates of the individual reagents and ensure that all the reagents have been stored as indicated on the product label. If the storage conditions were not strictly followed throughout, the results are not reliable, and the kit cannot be used anymore.
- Ensure that the calibrations of the pipettes used are up to date.
- Ensure that the cycler settings are as per the IFU.
Possible reasons and corrective actions for some of the findings during the assay are given in Table 9 below.
Table 9. APO-Easy genotyping kit troubleshooting recommendations.
|
Findings |
Possible reasons and corrective actions |
|
No calls in all channels for a sample |
Possibility: problems with the quality of gDNA or interference substances
Recommendation: extract again the sample and redo the qPCR. |
|
No signal in one or two channels |
Possibility: if for only one sample then redo the qPCR; if for all the samples, then check the expiration date of the kit and storage condition. |
|
Undetermined genotype for one SNP |
Possibility: the gDNA input was not sufficient.
Recommendation: Redo the test. During the same assay, for the same sample, use 2µL of sample’s gDNA with 23µL of master mix and 4µL of sample’s gDNA with 21µL of master mix. |
|
After retest, the genotype is still undetermined |
Possibility: presence of an interfering substance in the sample.
Recommendation: - The addition of K2EDTA in the PAXgene DNA tube might interfere with the sample’s genotyping, please check if EDTA were added in the tube or if the volume of blood sample was properly collected according to the manufacturer's instructions for use. - Triglycerides in the blood should not exceed the physiological concentration (normal blood range concentration: 0,5 - 1,5 g/L). If an excess of triglycerides is present in the blood sample, it might interfere with the correct genotyping of the patient. - Albumin should not exceed the physiological concentration (normal blood range concentration: 35 - 50 g/L). If the presence of albumin in the patient’s blood sample exceed this concentration, it might interfere with the genotyping results. If the problem persist, contact the technical assistance. |
|
Controls have no signal |
Confirm that the storage conditions and instructions given were followed. A pipetting problem might have occurred.
Recommendation: Redo the test. If you still do not have a signal, contact the technical assistance. |
|
Signal in NTC |
Possibility: the reaction mix were most probably contaminated with a template.
Recommendation: Redo the qPCR. If the problem persists, do not use the kit further and contact the technical assistance. |
8. Analytical Parameters
Accuracy: the APO-Easy® genotyping kit is 100% accurate in detecting APOE genotypes when compared to the reference methods (Sanger bidirectional sequencing and Next Generation Sequencing).
Sensitivity: the APO-Easy® genotyping assay detects 12,5 ng of human gDNA when used with the QuantStudio 5 Dx Real-Time PCR System.
Precision: the APO-Easy® genotyping kit is reproducible with 100% positive agreement results when testing different lots or when used by different operators.
Interferences: an excess of ethanol until 20%, an excess of bilirubin until 200 mg/mL, an excess of K2EDTA until 20 mg/mL, an excess of albumin until 25 g/L, an excess of hemoglobin until 100 g/L or an excess of triglycerides up to 18,2 g/L in the sample does not interfere with the genotyping results.
9. Clinical Performance
The APO-Easy genotyping kit meets its intended use as it has a 100% positive percent agreement when results are compared to Sanger bidirectional sequencing and Targeted Next-Generation Sequencing.
Table 10 APO-Easy genotyping test has 100% accuracy

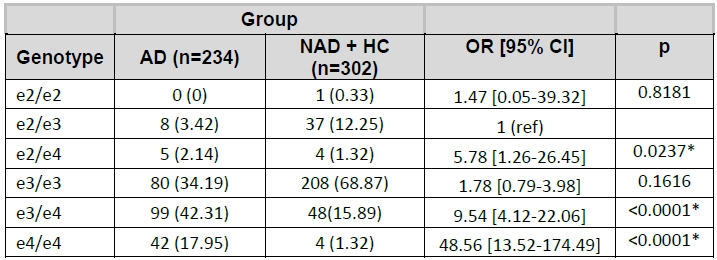
When the APOE genotype frequencies of Alzheimer’s Disease subjects were compared to those of Non -Alzheimer’s Disease and Healthy Control subjects combined, the presence of two copies of the ε4 allele (e4/e4 homozygote) increased the risk of AD 48.56 times compared to e2/e3. The presence of one copy of the ε4 allele increased the risk of AD 9.54 times and 5.78 times for e3/e4 heterozygotes and e2/e4 heterozygotes respectively when compared to e2/e3.
Table 11 Genotype based comparisons between AD and NAD + HC
10. Residual Risks and warnings
The APO-Easy® genotyping kit design and performance characteristics have been optimized by comprehensive testing in-house and in the hands of intended users, as verified when testing populations representative of the intended patient population. Firalis, the Manufacturing Company considers the remaining risks to users and patients minimal and acceptable for genetic testing.
- Negative results do not preclude health risks and should not be used as the sole basis for patient management decisions.
- Negative and Positive results must be combined with clinical observations, patient history, and other testing information.
The risks of using the APO-Easy Genotyping kit are minimal if test is used as intended. Users can further minimize risk by following the below recommendations:
Samples Contamination: contamination of samples with extraneous DNA or possible interferents can lead to inaccurate results. The use of clean workspaces, disposable gloves, and dedicated equipment for sample preparation will mitigate this residual risk.
Cross-contamination: improper handling of reagents or samples could result in cross-contamination between samples, leading to erroneous results. Maintain separate work areas for sample preparation, DNA extraction, and PCR setup, and work in a clean environment when handling pipettes and other equipment to prevent cross-contamination.
Results Readout: inexperienced users may misinterpret results leading to incorrect conclusions. The interpretation of the APO-Easy genotyping assay must be done by highly trained personnel to minimize any risk of incorrect results determination.
Inadequate Training: users with insufficient training or experience may be more prone to errors in assay setup or interpretation. We recommend the use of the APO-Easy test only to highly qualified personnel.
11. Limitations of the Procedure
- Reagents are not for use in therapeutic procedures.
- Not an automated method and not a self-test device.
- This assay is designed to quantify the APOE genotype from human PAXgene DNA tubes, within the assay quantification range.
- Results obtained from this assay must be interpreted within the context of all relevant clinical laboratory findings and are not to be used alone for any diagnosis.
- Contamination of genomic materials from external sources must be avoided by careful handling of the samples, kit reagents, and a clean working environment.
- Operators must avoid microbial contaminants during the procedures and should not use the kit components if evidence of microbial growth is observed
- This kit is intended to be used with the QS5-Dx Real-Time PCR System (ThermoFisher).
- Strict compliance with the recommendations given in the Assay Kit IFU is essential to obtain optimal results.
- Attention must be paid to expiration dates and storage conditions for each box in the kit. Do not use it if expired or if incorrectly stored.
- Do not mix or substitute reagents with those from other lots or sources.
- Use only certified nuclease-free tips and microcentrifuge tubes.
- Use Nuclease-free water stored in clean containers.
- Any variation in the process described in the IFU can cause variations in the result.
- APO-Easy® genotyping is not intended to predict or detect response to therapy, or to help select the optimal therapy for patients.
12. Chemical safety guidelines
To minimize the hazards of chemicals:
- Read and understand the Safety Data Sheets (SDSs) provided by the chemical manufacturer before you store, handle, or work with any chemicals or hazardous materials.
- Avoid direct contact with chemicals.
- Wear appropriate personal protective equipment when handling chemicals such as safety glasses, gloves, and protective clothing.
- Avoid the inhalation of chemicals. Do not smoke nor leave the chemical containers open. Use only with adequate ventilation or a fume hood.
- For additional safety guidelines, consult the SDS.
- Check regularly for chemical leaks or spills. If a leak or spill occurs, follow the manufacturer’s cleanup procedures as recommended in the SDS.
- Comply with all local, state/provincial, or national laws and regulations related to chemical storage, handling, and disposal.
13. Technical hints
- For technical assistance related to DNA extraction refer to the datasheet provided with the recommended QIAamp DSP DNA blood mini-Kit (ref: 61104) by Qiagen.
- Avoid any contamination among samples and reagents. For this purpose, change tips at each step. Bacterial or fungal contamination in any reagents may cause erroneous results.
- Dispose of consumable materials and unused contents in appropriate containers.
- The procedure is only suitable for use with whole blood from PAXgene DNA tubes.
14. Liability
- This kit is only intended for the in vitro determination of APOE genotype in human whole blood from PAXgene DNA tubes.
- This kit is only intended for use by qualified personnel.
- Firalis shall not be responsible for any damage or loss due to using the kit in any way other than as expressly stated in these instructions.
- Firalis is not responsible for any patent infringements that might result from the use or derivation of this product.
Technical assistance
For technical assistance, call Firalis SA Technical Services at +33-389 911 320 or visit the Firalis SA website at http://www.firalis.com or contact “contact@firalis.com”.
Serious incident report notice
A serious incident is the cause of any malfunction or deterioration in the characteristics or performance of a device, and can be classed in at least one of the following consequences:
• The death of a patient, user or other person
• A temporary or permanent serious deterioration in a patient's, user's or other person's state of health
• A serious public health threat.
In case of doubt, users should always report.
If you conclude that a reportable incident is involved, please contact the manufacturer at clinical@firalis.com. You can also find the contact information of the competent authority in your country here: https://health.ec.europa.eu/medical-devices-sector/new-regulations/contacts_en.
When reporting a serious incident please compile the following information:
- Trade name of the device (TM for Trade Mark, © for Copyright)
- Name and address of the manufacturer
- Lot number
-Serial number
-UDI (Unique Device Identification) Code
-An accurate and concise description of the serious incident and the serious or possibly serious consequences for the patient, user or a third party.
The device concerned should not be disposed of but should be made available to the manufacturer for further analysis to determine the causes of the incident. You can add your return shipment request when contacting the manufacturer for reporting the incident.
Summary of Safety and Performance
The Summary of Safety and Performance of the APO-Easy® Genotyping kit has been validated by the Notified Body 2797 (BSI group) and is available to the public on the European database on medical devices (Eudamed) website by using the UDI-DI number of the APO-Easy® Genotyping kit in the database. This identifier is available with the QR code placed on the kit packaging label.
The Summary of Safety and Performance will be regularly updated with any relevant information concerning the APO-Easy® Genotyping kit.
References
- Marias, A.D. Apolipoprotein E in lipoprotein metabolism, health, and cardiovascular disease. Pathology 2019, 51, 165–176.
- Semaev, S.; Shakhtshneider, E.; Shcherbakova, L.; Ivanoshchuk, D.; Orlov, P.; Malyutina, S.; Gafarov, V.; Ragino, Y.; Voevoda, M. Associations of APOE Gene Variants rs429358 and rs7412 with Parameters of the Blood Lipid Profile and the Risk of Myocardial Infarction and Death in a White Population of Western Siberia. Curr. Issues Mol. Biol. 2022, 44, 1713-1724. https://doi.org/10.3390/cimb44040118.
- Davis AA et al (2020) APOE genotype regulates pathology and disease progression in synucleinopathy. Sci Transl Med:12(529)
- Mishra A, Ferrari R, Heutink P, Hardy J, Pijnenburg Y, Posthuma D, et al. Gene-based association studies report genetic links for clinical subtypes of frontotemporal dementia. Brain. 2017;140(5):1437–46.
- Su WH, Shi ZH, Liu SL, Wang XD, Liu S, Ji Y. Updated meta-analysis of the role of APOE ε2/ε3/ε4 alleles in frontotemporal lobar degeneration. Oncotarget. 2017 Jul 4;8(27):43721-43732. doi: 10.18632/oncotarget.17341.
- Ohm, T., Scharnagl, H., März, W. et al. Apolipoprotein E isoforms and the development of low and high Braak stages of Alzheimer’s disease-related lesions. Acta Neuropathol 98, 273–280 (1999). https://doi.org/10.1007/s004010051080.
- Yamazaki, Y., Zhao, N., Caulfield, T.R. et al. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat Rev Neurol 15, 501–518 (2019). https://doi.org/10.1038/s41582-019-0228-7.
- Mayeux R, Saunders AM, Shea S, Mirra S, Evans D, Roses AD, et al. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer’s disease. Alzheimer's disease centers consortium on Apolipoprotein E and Alzheimer's disease. N Engl J Med. 1998;338(8):506–11.
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993 Aug 13;261(5123):921-3. doi: 10.1126/science.8346443. PMID: 8346443.
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9649-53. doi: 10.1073/pnas.90.20.9649.
- Strittmatter, W. J., Saunders, A. M., Schmechel, D., Pericak-Vance, M., Enghild, J., Salvesen, G. S., et al. (1993). Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 90, 1977–1981. doi: 10.1073/pnas.90.5.1977
- Farrer LA, Cupples LA, Haines JL, et al. Effects of Age, Sex, and Ethnicity on the Association Between Apolipoprotein E Genotype and Alzheimer Disease: A Meta-analysis. JAMA. 1997;278(16):1349–1356. doi:10.1001/jama.1997.03550160069041.
- Bu G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009 May;10(5):333-44. doi: 10.1038/nrn2620.
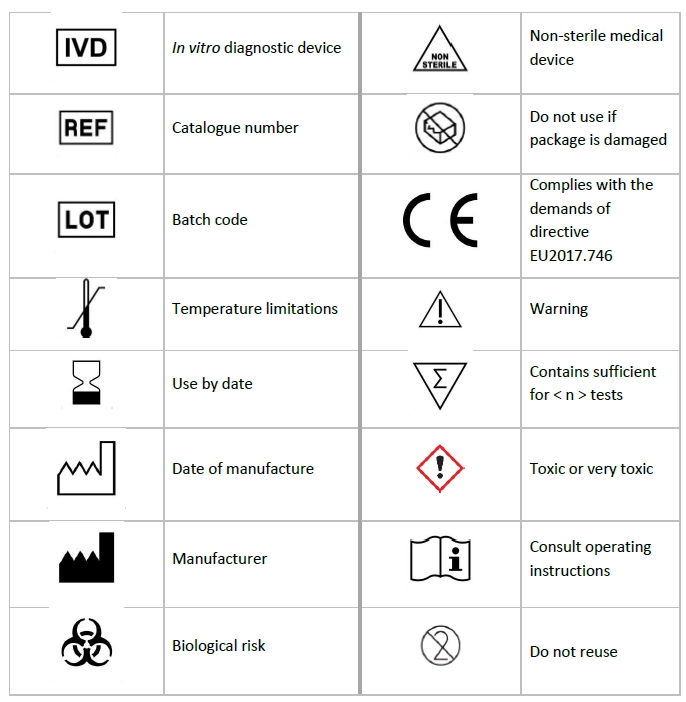
Symbol legends used in the IFU and labels of the kit

Thank you for choosing Firalis
For any further information, MSDS or
other languages Instructions enquiries, please write us:
